Each production site of thoroughly implements stringent quality control at every stage of the process, from procuring raw materials to production. We will propose to you a production site to best meet your needs of cost, content of items to be produced, etc.
-


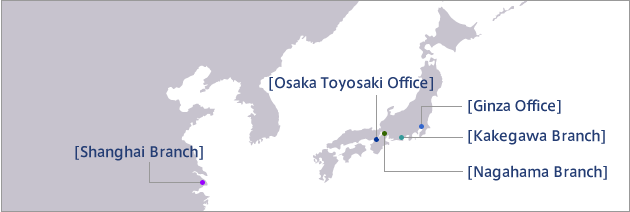
A comprehensive site designed to realize organic coordination of every function by connecting office buildings, the production wing, and the logistics wing with walkways within the 90,000 ㎡ premises.
From basic skincare to makeup items. Kakegawa Branch is a comprehensive site in Japan boasting a diverse production lineup. Recommended for production of high-end brand products as well as items marketed overseas with “Made in Japan” credibility. The unique exterior is designed after an atelier that hand-makes each and every cosmetic item.

-


Multi-purpose plant compliant with GMP (Good Manufacturing Practice) standards for pharmaceuticals. Handles production of cosmetics as well as pharmaceuticals, quasi-drugs, and functional foods.
Maintains an extremely clean environment of “Class 10000” (10,000 dust particles per 1 ㎥ for the entire bulk production area. The pharmaceuticals manufacturing space complies with GMP for pharmaceuticals set by the Ministry of Health, Labor and Welfare. An additional advantage is the site’s ability to handle small-lot production.

-


The Shanghai Branch is a production site in China with Japanese staff, thoroughly implementing stringent quality control equaling that at each Japanese site.
Supplies high quality products efficiently by utilizing local materials and human resources. The bulk manufacturing facility, the bedrock of cosmetics manufacturing, is made in Japan for reliability. Also, we have entered into a partnership with a local Japanese corporation so as to enhance management and operational credibility. Products manufactured in bulk in Japan and filled in Shanghai can be labeled “Made in Japan.”